
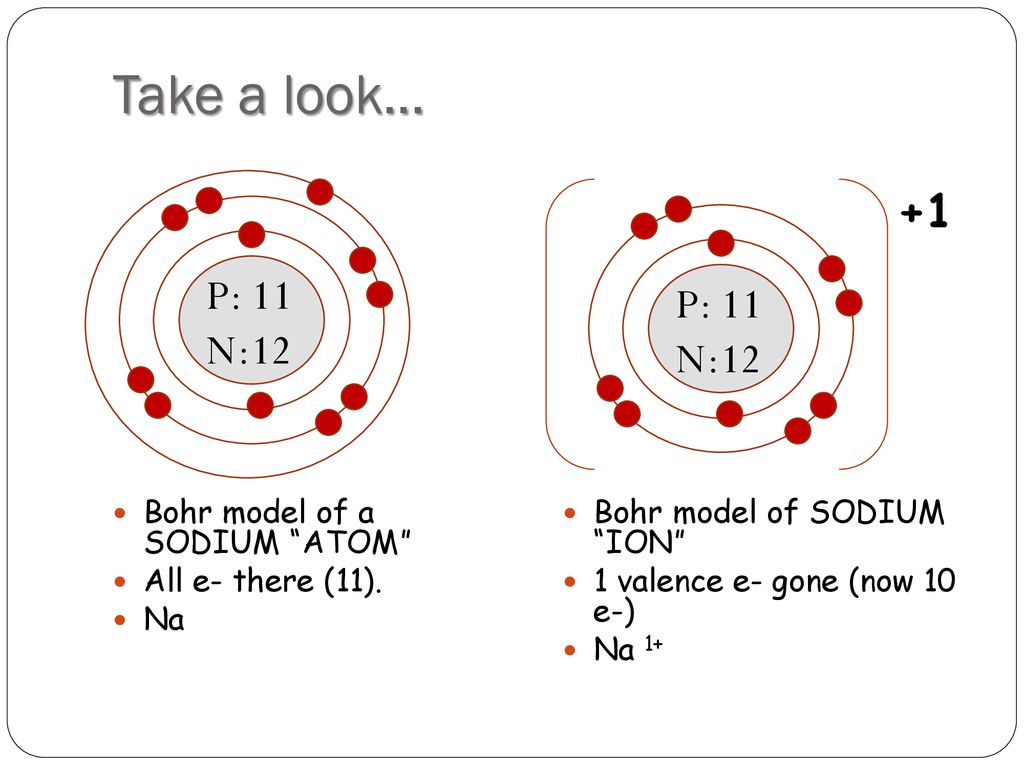
Each orbit (or shell) can hold a certain number. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. This is similar to the planets revolving around the sun, except that the orbits are non-planar. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. In this case, the electron starts out with \(n = 4\), so \(n_1 = 4\). Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Bohr’s model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.
What is the energy (in joules) and the wavelength (in meters) of the line in the spectrum of hydrogen that represents the movement of an electron from Bohr orbit with n = 4 to the orbit with n = 6? In what part of the electromagnetic spectrum do we find this radiation? In 1913, a Danish physicist, Niels Bohr (18851962 Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. It holds a special place in history as it gave. Bohr’s Model of the hydrogen atom attempts to plug in certain gaps as suggested by Rutherford’s model. Niels Bohr introduced the atomic Hydrogen model in the year 1913. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. Bohr model of the hydrogen atom was the first atomic model to successfully explain the radiation spectra of atomic hydrogen. \): Calculating Electron Transitions in a One–electron System The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model.



 0 kommentar(er)
0 kommentar(er)
